Introduction: Isatuximab (Isa) is an immunoglobulin (Ig)G1 monoclonal antibody targeting a CD38 transmembrane glycoprotein in multiple myeloma (MM). Isa is approved for use in multiple countries to treat adults with relapsed/refractory MM (RRMM) when given in combination with either pomalidomide-dexamethasone (Pd) or carfilzomib-dexamethasone (Kd). Hypogammaglobulinemia (HGG; polyclonal IgG <4 g/L) and the associated infection risk is a potential complication for patients (pts) with MM treated with immunotherapies. High infection and HGG rates have been reported during treatment (tx) with antimyeloma therapies, and in particular, recently, with anti-B cell maturation antigen-directed therapies. 1,2 Here, we explore the impact of Isa monotherapy on Ig levels, and of approved Isa combinations (Isa-Pd and Isa-Kd) on Ig levels and the incidence, severity, and clinical significance of HGG, including the incidence of infections depending on concomitance of HGG or not, in pts with RRMM.
Methods: Pts with RRMM with central lab Ig data from 3 monotherapy studies were pooled (across dose levels, most >10 mg/kg) to retrospectively explore Ig levels throughout tx with Isa monotherapy until disease progression, intolerable toxicity, or withdrawal.We performed a side-by-side analysis of pts from the Phase 3 ICARIA-MM (Isa-Pd vs Pd) and IKEMA (Isa-Kd vs Kd) studies with/without HGG at baseline and concomitance of HGG with infections. Pts were analyzed overall and according to the heavy chain of the monoclonal protein at study entry, where IgG consisted of monoclonal IgG (M-spike in g/dL *1000) subtracted from total quantitative IgG (mg/dL) to obtain the true polyclonal IgG level, and non-IgG consisted of other Ig or light chain subtypes. Recovery was defined as >6 g/L (complete) or ≥4 g/L (partial) after having <4 g/L at any time.
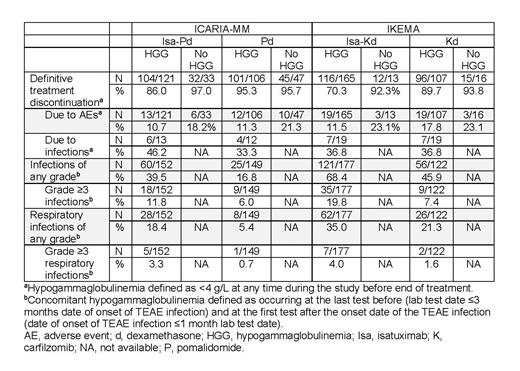
Results: Isa monotherapy analyses revealed a fast decrease in non-involved IgA levels after 1 month, followed by stabilization, which has also been reported with daratumumab monotherapy. Isa monotherapy did not induce decreased IgG levels. In ICARIA-MM, more pts with non-IgG vs IgG MM at study entry had HGG during tx (54.0% vs 14.4%, Isa-Pd; 39.6% vs 12.7%, Pd), whereas incidences were similar in IKEMA (11.7% vs 8.9%, Isa-Kd; 15.5% vs 11.4%, Kd). A smaller proportion of pts with HGG vs without HGG at any time during the study had definitive tx discontinuation in both ICARIA-MM and IKEMA; discontinuations related to adverse events (AEs) were less frequent in pts with HGG vs without HGG (Table). Of these AEs, infections led to discontinuation in 33.3% to 46.2% of patients with HGG at any time, regardless of tx (Table). In ICARIA-MM, 70.9% (Isa-Pd) and 55.5% (Pd) of pts without HGG at baseline developed HGG post-baseline; in IKEMA, 90.6% (Isa-Kd) and 82.0% (Kd) developed HGG post-baseline. Among pts who received Isa-Pd, those who had IgG MM at study entry experienced a larger proportion of recovery (9.1%, complete; 26.0%, partial) than those with non-IgG MM (6.8%, complete; 15.9%, partial); similar results were seen with Pd in pts with (21.9%, complete; 40.6%, partial) and without IgG MM (4.8%, complete; 19.0%, partial). Pts who had IgG MM at study entry and received Isa-Kd experienced a larger proportion of recovery (17.0%, complete; 33.0%, partial) than those with non-IgG MM (3.8%, complete; 7.5%, partial); similar results were seen with Kd in pts with (14.7%, complete; 25.3%, partial) and without IgG MM (3.1%, complete; 3.1%, partial). Infections of any grade concomitant with HGG were reported in 28.2% of pts in ICARIA-MM and 59.2% in IKEMA, with a larger proportion of pts receiving Isa combinations reporting infections of any grade and Grade ≥3 (Table). Respiratory infections of any grade concomitant with HGG were more frequent in pts receiving Isa-Pd vs Pd and Isa-Kd vs Kd (Table). There were low proportions of pts with Grade ≥3 respiratory infections concomitant with HGG regardless of tx arm (Table).
Conclusions: Monotherapy with Isa had little impact on Ig levels. Among pts treated with Isa monotherapy, there is an early drop in non-involved IgA levels, followed by stabilization. In ICARIA-MM and IKEMA, HGG did not contribute to increased Grade ≥3 infections. Isa added to backbone tx is safe with a low rate of Grade ≥3 infections despite the decrease in Ig levels.
Funding: Sanofi.
References:
1. Lancman G, et al. Blood. 2022;140:10073-4.
2. Rodriguez-Otero P, et al. J Clin Oncol. 2023;41:8020.
Disclosures
Rodriguez:Janssen, Takeda, Bristol Myers Squibb, Amgen, Karyopharm Therapeutics: Membership on an entity's Board of Directors or advisory committees. Perrot:Abbvie, Adaptive, Amgen, BMS, Janssen, Pfizer, Sanofi, Takeda: Honoraria. Richardson:Oncopeptides: Consultancy, Research Funding; GSK: Consultancy; Takeda: Research Funding; Karyopharm: Consultancy, Research Funding; Sanofi: Consultancy; AstraZeneca Pharmaceuticals LP, Bristol-Myers, Squibb Company, Celgene Corporation, GlaxoSmithKline, Janssen Biotech Inc, Karyopharm Therapeutics, Oncopeptides, Sanofi, Secura Bio, Takeda Pharmaceuticals USA Inc;: Consultancy; Bristol Myers Squibb: Consultancy, Other: Contracted research, Research Funding. Spicka:Janssen-Cilag: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events; Support for attending meetings and/or travel events; Novartis: Other: Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Other: Received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events; Participation on a Data Safety Monitoring Board or Advisory Board; GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events; Support for attending meetings and/or travel events; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events; Support for attending meetings and/or travel events; Celgene: Consultancy, Other: Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events; Support for attending meetings and/or travel events; Bristol Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events; Support for attending meetings and/or travel events. Oriol:BMS/Celgene: Consultancy, Honoraria, Speakers Bureau; GSK: Consultancy, Honoraria, Speakers Bureau; Amgen: Consultancy, Other: Consulting fees. Suzuki:Amgen: Other: Remuneration for lecture , Research Funding; Abbvie GK: Other: Remuneration for lecture ; Bristol Myers Squibb: Other: Remuneration for lecture, Research Funding; Novartis International AG: Other: Remuneration for lecture; ONO PHARMACEUTICAL CO., LTD.: Other: Remuneration for lecture; Sanofi S.A: Other: Remuneration for lecture; Takeda Pharmaceutical Company Limited: Other: Remuneration for lecture, Research Funding; Janssen Pharmaceutical K.K.: Other: Remuneration for lecture. Crusoe:Janssen: Research Funding; Amgen, BMS, Janssen, Sanofi, Takeda: Honoraria. Armstrong:Sanofi: Current Employment. Tekle:Sanofi: Current Employment. Risse:Sanofi: Current Employment. Moreau:GSK: Honoraria, Other: Advisory Board; janssen, celgene BMS, abbvie, sanofi, amgen, takeda, pfizer: Honoraria, Other: advisory boards.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal